| Structure | Name/CAS No. | Articles |
|---|---|---|
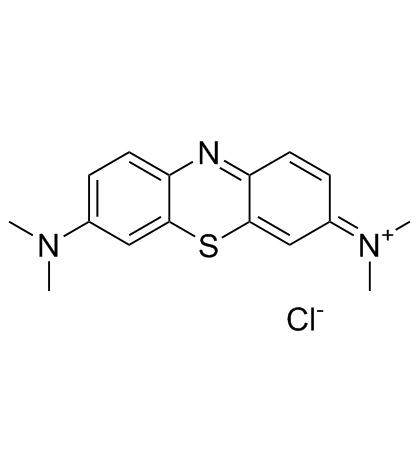 |
Methylene Blue
CAS:61-73-4 |
|
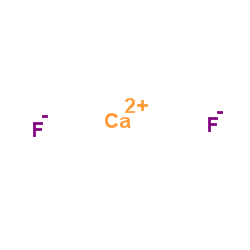 |
Calcium fluoride
CAS:7789-75-5 |