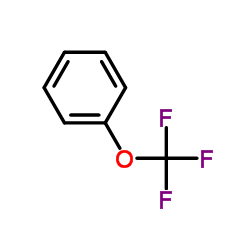
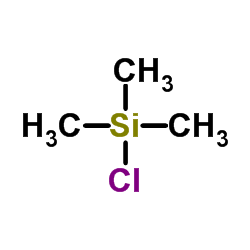
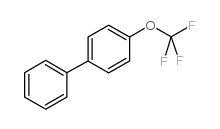
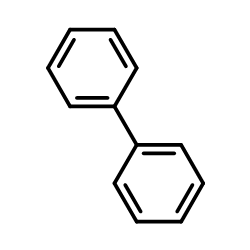
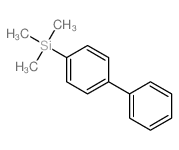
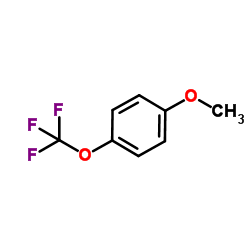
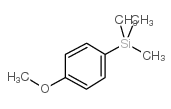
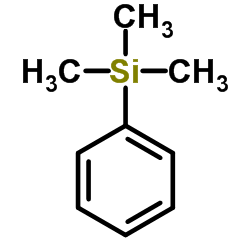
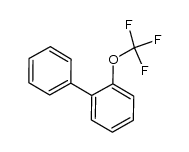
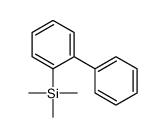
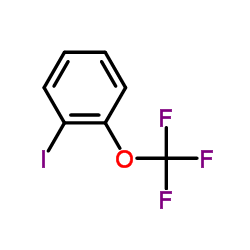
|
~51% |
|
~30% |
|
~57% |
|
~81% |
|
~75% |
|
~71% |
|
~96% |