| Structure | Name/CAS No. | Articles |
|---|---|---|
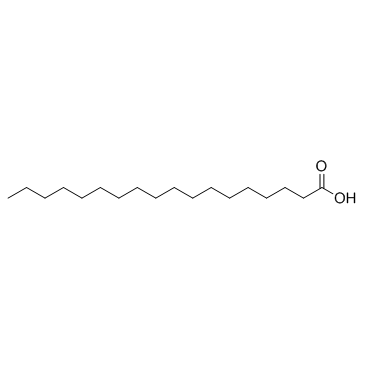 |
stearic acid
CAS:57-11-4 |
|
 |
Retinoic acid
CAS:302-79-4 |
|
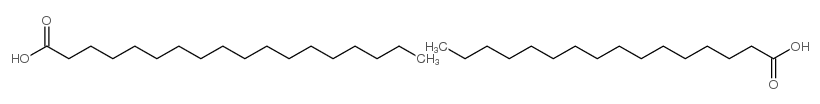 |
Parteck LUB STA 50
CAS:67701-03-5 |