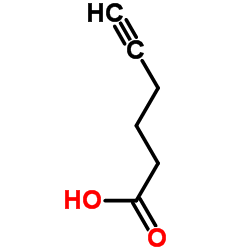
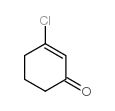
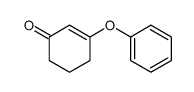
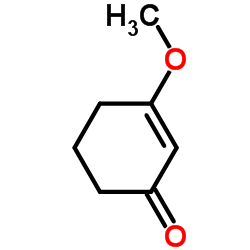
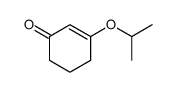
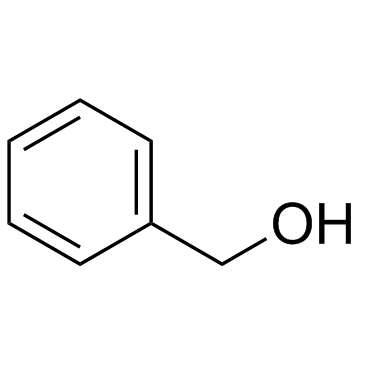
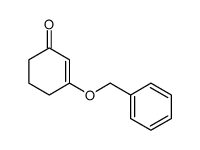
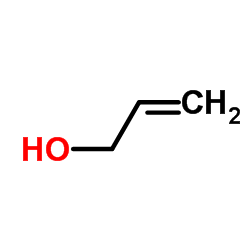
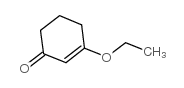
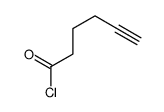
|
~75% |
|
~63% |
|
~74% |
|
~74% |
|
~58% |
|
~90% |
|
~79% |
|
~69% |