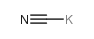|
~81% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |


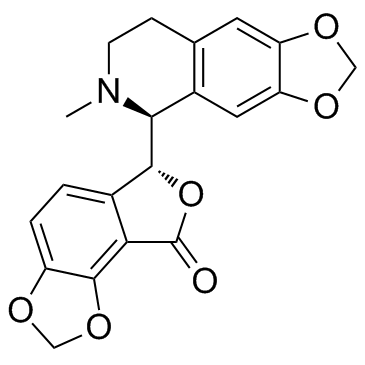
![Furo[3,4-e]-1,3-benzodioxole-6-carboxylicacid, 6,8-dihydro-8-oxo Structure](https://image.chemsrc.com/caspic/411/64395-07-9.png)
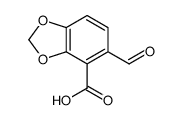
![N-(2-(benzo[d][1,3]dioxol-5-yl)ethyl)-8-oxo-6,8-dihydro-[1,3]dioxolo[4,5-e]isobenzofuran-6-carboxamide Structure](https://image.chemsrc.com/caspic/381/185022-35-9.png)