| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
2-AMINO-4,6-DINITROTOLUENE
CAS:25501-32-0 |
|
 |
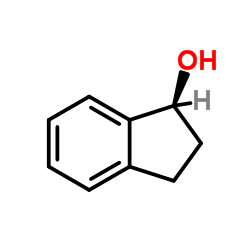
(R)-(-)-1-INDANOL
CAS:697-64-3 |
|
 |
2,3-Dihydro-1H-inden-1-ol
CAS:6351-10-6 |