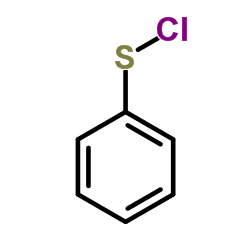
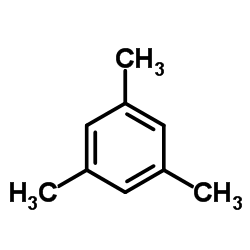
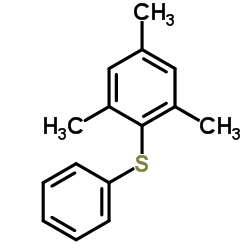
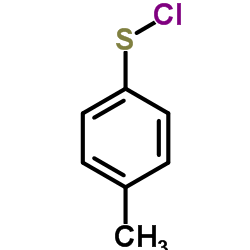
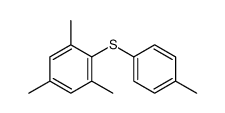
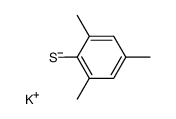
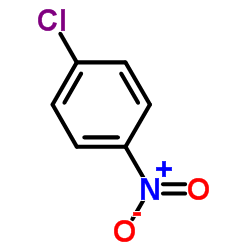
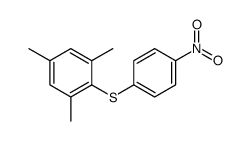
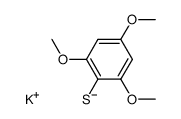
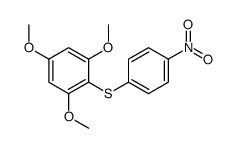
|
~86% |
|
~77% |
|
~78% |
|
~74% |