| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodium chloride
CAS:7647-14-5 |
|
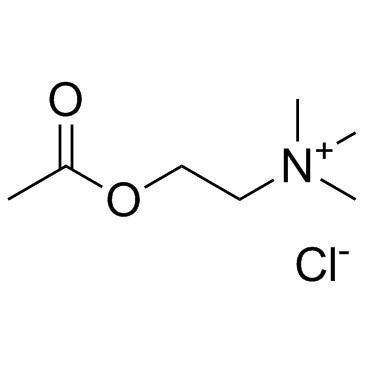 |
Acetylcholine chloride
CAS:60-31-1 |
|
 |
magnesium sulfate
CAS:7487-88-9 |
|
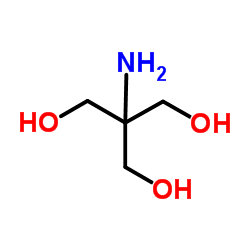 |
Trometamol
CAS:77-86-1 |
|
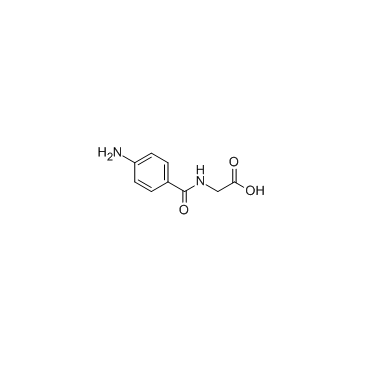 |
4-Aminohippuric acid
CAS:61-78-9 |
|
 |
SODIUM CHLORIDE-35 CL
CAS:20510-55-8 |
|
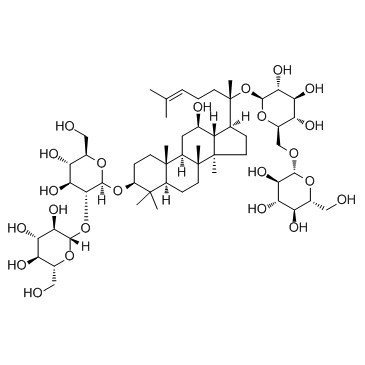 |
ginsenoside Rb1
CAS:41753-43-9 |
|
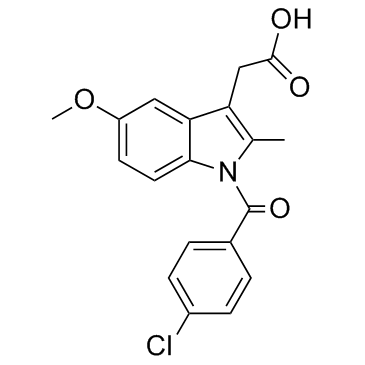 |
Indometacin
CAS:53-86-1 |
|
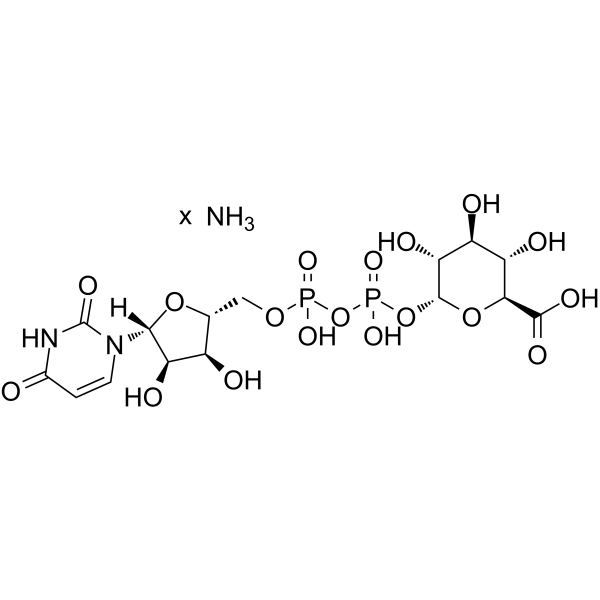 |
Uridine diphosphate glucuronic acid ammonium
CAS:43195-60-4 |
|
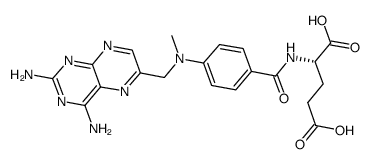 |
L(+)-Amethopterin hydrate
CAS:133073-73-1 |