Nabilone for the treatment of medication overuse headache: results of a preliminary double-blind, active-controlled, randomized trial.
Luigi Alberto Pini, Simona Guerzoni, Maria Michela Cainazzo, Anna Ferrari, Paola Sarchielli, Ilaria Tiraferri, Michela Ciccarese, Maurizio Zappaterra
Index: J. Headache Pain 13(8) , 677-84, (2012)
Full Text: HTML
Abstract
Medication overuse headache (MOH) is a severe burden to sufferers and its treatment has few evidence-based indications. The aim of this study is to evaluate efficacy and safety of nabilone in reducing pain and frequency of headache, the number of analgesic intake and in increasing the quality of life on patients with long-standing intractable MOH. Thirty MOH patients were enrolled at the University of Modena's Interdepartmental Centre for Research on Headache and Drug Abuse (Italy) in a randomized, double-blind, active-controlled, crossover study comparing nabilone 0.5 mg/day and ibuprofen 400 mg. The patients received each treatment orally for 8 weeks (before nabilone and then ibuprofen or vice versa), with 1 week wash-out between them. Randomization and allocation (ratio 1:1) were carried out by an independent pharmacy through a central computer system. Participants, care givers, and those assessing the outcomes were blinded to treatment sequence. Twenty-six subjects completed the study. Improvements from baseline were observed with both treatments. However, nabilone was more effective than ibuprofen in reducing pain intensity and daily analgesic intake (p < 0.05); moreover, nabilone was the only drug able to reduce the level of medication dependence (-41 %, p < 0.01) and to improve the quality of life (p < 0.05). Side effects were uncommon, mild and disappeared when nabilone was discontinued. This is the first randomized controlled trial demonstrating the benefits of nabilone on headache, analgesic consumption and the quality of life in patients with intractable MOH. This drug also appears to be safe and well-tolerated. Larger scale studies are needed to confirm these preliminary findings.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
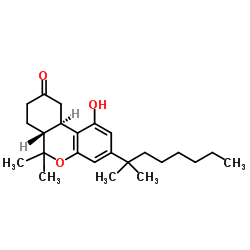 |
(−)-nabilone
CAS:51022-71-0 |
C24H36O3 |
|
Oral nabilone capsules in the treatment of chemotherapy-indu...
2008-01-01 [Expert Opin. Investig. Drugs 17(1) , 85-95, (2008)] |
|
A randomized, double-blinded, crossover pilot study assessin...
2010-05-01 [Arch. Phys. Med. Rehabil. 91(5) , 703-7, (2010)] |
|
An open-label comparison of nabilone and gabapentin as adjuv...
2011-01-01 [Pain Pract. 11(4) , 353-68, (2011)] |
|
Subjective, cognitive and cardiovascular dose-effect profile...
2013-09-01 [Addict. Biol. 18(5) , 872-81, (2013)] |
|
Cannabinoids in the treatment of chemotherapy-induced nausea...
2012-04-01 [J. Natl. Compr. Canc. Netw. 10(4) , 487-92, (2012)] |