| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Piroxicam
CAS:36322-90-4 |
|
 |
Aspirin
CAS:50-78-2 |
|
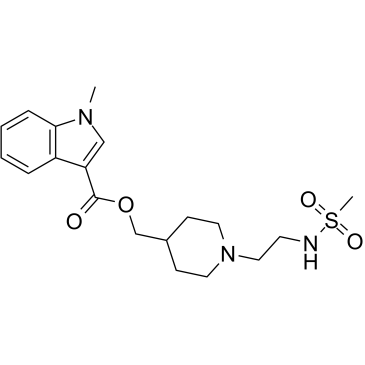 |
GR113808
CAS:144625-51-4 |