| Structure | Name/CAS No. | Articles |
|---|---|---|
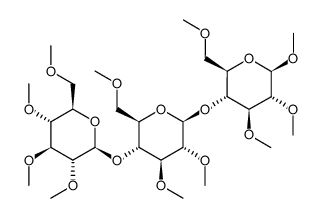 |
methyl cellulose
CAS:9004-67-5 |
|
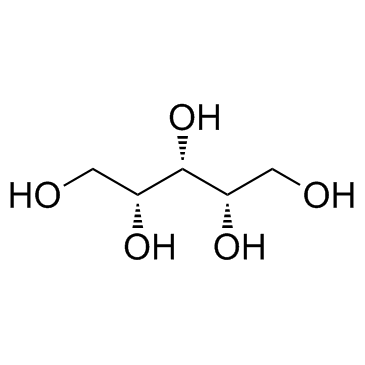 |
Xylitol
CAS:87-99-0 |
|
 |
Dicalcium phosphate
CAS:7757-93-9 |
|
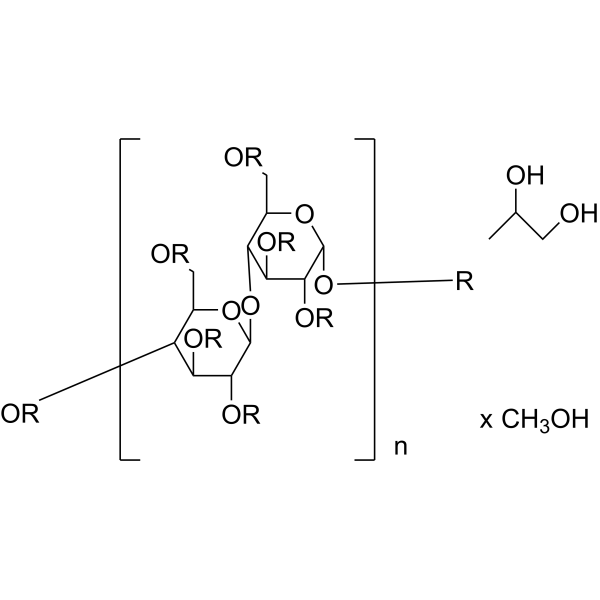 |
Hydroxypropyl Methyl Cellulose
CAS:9004-65-3 |
|
 |
Lactose
CAS:63-42-3 |