The palladium-catalyzed trifluoromethylation of aryl chlorides.
Eun Jin Cho, Todd D Senecal, Tom Kinzel, Yong Zhang, Donald A Watson, Stephen L Buchwald
Index: Science 328 , 1679-1681, (2010)
Full Text: HTML
Abstract
The trifluoromethyl group can dramatically influence the properties of organic molecules, thereby increasing their applicability as pharmaceuticals, agrochemicals, or building blocks for organic materials. Despite the importance of this substituent, no general method exists for its installment onto functionalized aromatic substrates. Current methods either require the use of harsh reaction conditions or suffer from a limited substrate scope. Here we report the palladium-catalyzed trifluoromethylation of aryl chlorides under mild conditions, allowing the transformation of a wide range of substrates, including heterocycles, in excellent yields. The process tolerates functional groups such as esters, amides, ethers, acetals, nitriles, and tertiary amines and, therefore, should be applicable to late-stage modifications of advanced intermediates. We have also prepared all the putative intermediates in the catalytic cycle and demonstrated their viability in the process.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
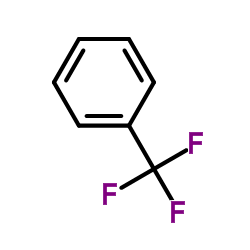 |
Benzotrifluoride
CAS:98-08-8 |
C7H5F3 |
|
Utilizing capillary gas chromatography mass spectrometry to ...
2003-02-05 [J. Pharm. Biomed. Anal. 31(1) , 63-74, (2003)] |
|
Study of the voltammetric behaviour of the ethalfluralin and...
2004-01-01 [Environ. Monit. Assess. 151(1-4) , 9-18, (2009)] |
|
Precursor chemistry matters in boosting photoredox activity ...
2015-11-21 [Nanoscale 7 , 18062-70, (2015)] |
|
Accelerated separation of GC-amenable lipid classes in plant...
2015-12-01 [Anal. Bioanal. Chem 407 , 9019-28, (2015)] |
|
An integrated microreactor system for self-optimization of a...
2010-09-17 [Angew. Chem. Int. Ed. Engl. 49(39) , 7076-80, (2010)] |