| Structure | Name/CAS No. | Articles |
|---|---|---|
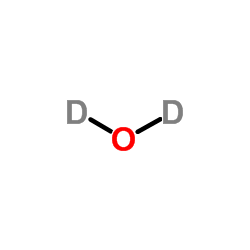 |
Heavy water
CAS:7789-20-0 |
|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
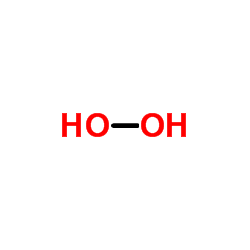 |
Hydrogen peroxide
CAS:7722-84-1 |
|
 |
N,N-Dimethylformamide
CAS:68-12-2 |
|
 |
3-Ethyl-2,4-pentanedione
CAS:1540-34-7 |
|
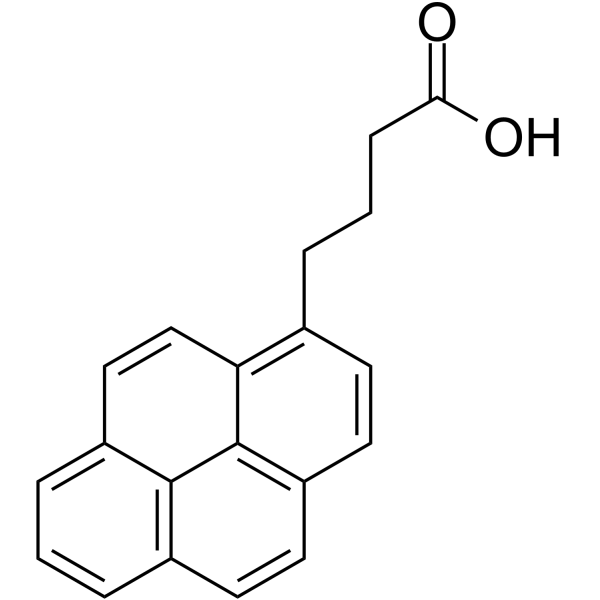 |
1-Pyrenebutyric Acid
CAS:3443-45-6 |
|
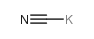 |
POTASSIUM CYANIDE
CAS:151-50-8 |
|
 |
MES
CAS:4432-31-9 |