| Structure | Name/CAS No. | Articles |
|---|---|---|
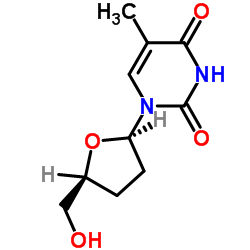 |
2',3'-Dideoxythymidine
CAS:3416-05-5 |
|
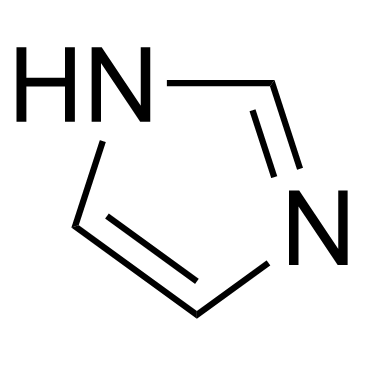 |
Imidazole
CAS:288-32-4 |
|
 |
Acetonitrile
CAS:75-05-8 |
|
 |
Methanol
CAS:67-56-1 |
|
 |
methylamine
CAS:74-89-5 |
|
 |
Dichloromethane
CAS:75-09-2 |
|
 |
Ethanoic anhydride
CAS:108-24-7 |
|
 |
Water
CAS:7732-18-5 |
|
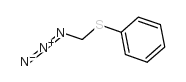 |
Azidomethyl Phenyl Sulfide
CAS:77422-70-9 |
|
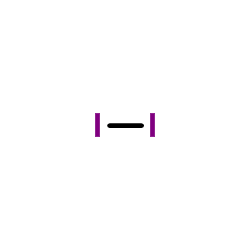 |
molecular iodine
CAS:7553-56-2 |