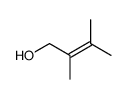
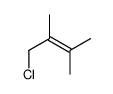
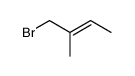
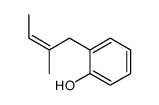
|
~59% |
|
~75% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~89% |
|
~% |
|
~% |
|
~73% |
|
~% |
|
~% |
|
~61% |
|
~% |
|
~86% |
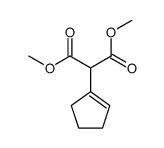
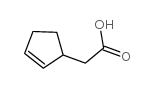




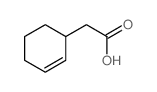

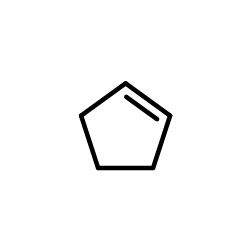
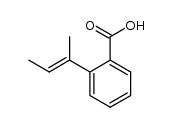
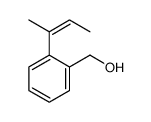
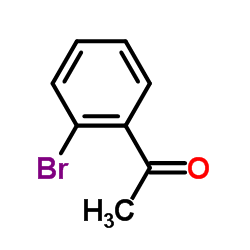
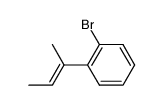
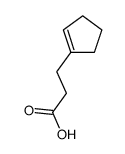
![1-oxaspiro[4.4]non-8-en-2-one Structure](https://image.chemsrc.com/caspic/316/86971-90-6.png)