[Triamcinolone acetonide in the treatment of corticosteroid-resistant asthma: risks and benefits].
César Picado, María del Carmen Vennera
Index: Arch. Bronconeumol. 44(6) , 324-7, (2008)
Full Text: HTML
Abstract
Few therapeutic alternatives to prednisone are available for severe, corticosteroid-resistant asthma. Injectable triamcinolone acetonide (TA) has been used in this type of asthma, although its use is controversial. TA shows considerable efficacy when compared with prednisone according to nearly all studies, although the majority do not provide a high level of evidence. The use of TA has been questioned, with claims put forward that it is equivalent to increasing the corticosteroid dose, thus leading to a higher risk of adverse effects. This would mean that TA would not represent an improvement over prednisone because of the trade-offs between risks and benefits. This interpretation is questionable, however, because the data show that TA causes fewer adverse effects than prednisone, meaning that the balance of risks and benefits does favor TA. Therefore, TA can be considered a useful option for the treatment of patients with severe prednisone-resistant asthma.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
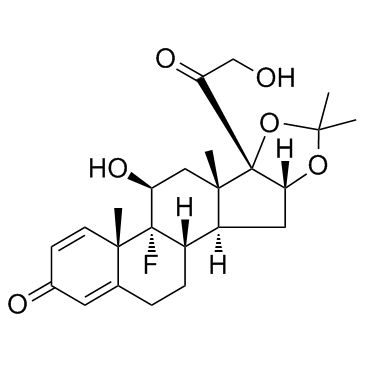 |
Triamcinolone acetonide
CAS:76-25-5 |
C24H31FO6 |
|
Novel Nrf2 activators from microbial transformation products...
2015-03-01 [Br. J. Pharmacol. 172(5) , 1237-49, (2015)] |
|
Evaluation of the reporting level to detect triamcinolone ac...
2015-01-01 [J. Steroid Biochem. Mol. Biol. 145 , 94-102, (2015)] |
|
A general analytical platform and strategy in search for ill...
2014-11-01 [J. Pharm. Biomed. Anal. 100 , 215-29, (2014)] |
|
Detection and characterization of triamcinolone acetonide me...
2014-08-30 [Rapid Commun. Mass Spectrom. 28(16) , 1829-39, (2015)] |
|
Verteporfin therapy and triamcinolone acetonide: convergent ...
2006-01-01 [Eur. J. Ophthalmol. 16(6) , 824-34, (2006)] |