Pharmacokinetics of triamcinolone acetonide for the treatment of macular edema.
Taygan Yilmaz, Miguel Cordero-Coma, Thomas J Federici
Index: Expert Opin. Drug Metab. Toxicol. 7(10) , 1327-35, (2011)
Full Text: HTML
Abstract
The use of intravitreal triamcinolone acetonide (TA) for the treatment of various types of macular edema has been widespread, particularly for the last decade. Currently, there is a scant amount of evidence-based literature evaluating the pharmacokinetic profile of TA despite clinical data showing the efficacy of intravitreal TA for multiple forms of macular edema.This paper is an extensive review of human and experimental studies published on the pharmacokinetics of TA for the treatment of macular edema. The literature search was conducted via OVID, TRIP Database and EMBASE, up to April 2011.The pharmacokinetic profile of TA is unpredictable and the agent has a time-limited therapeutic action due to its relatively short half-life. This has led to the need for repeated injections. Future research should investigate the pharmacokinetic profiles of TA when administered intravitreally, as well as through alternate routes in more robust studies.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
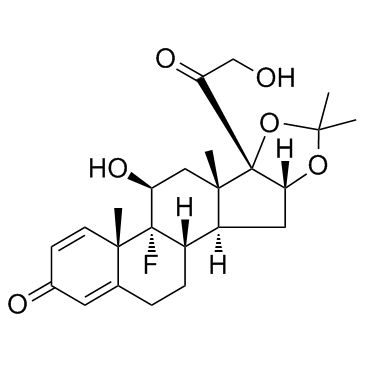 |
Triamcinolone acetonide
CAS:76-25-5 |
C24H31FO6 |
|
Novel Nrf2 activators from microbial transformation products...
2015-03-01 [Br. J. Pharmacol. 172(5) , 1237-49, (2015)] |
|
Evaluation of the reporting level to detect triamcinolone ac...
2015-01-01 [J. Steroid Biochem. Mol. Biol. 145 , 94-102, (2015)] |
|
A general analytical platform and strategy in search for ill...
2014-11-01 [J. Pharm. Biomed. Anal. 100 , 215-29, (2014)] |
|
Detection and characterization of triamcinolone acetonide me...
2014-08-30 [Rapid Commun. Mass Spectrom. 28(16) , 1829-39, (2015)] |
|
Verteporfin therapy and triamcinolone acetonide: convergent ...
2006-01-01 [Eur. J. Ophthalmol. 16(6) , 824-34, (2006)] |