Enhancing the photoluminescence of polymer-stabilized CdSe/CdS/ZnS core/shell/shell and CdSe/ZnS core/shell quantum dots in water through a chemical-activation approach.
Mingfeng Wang, Meng Zhang, Jieshu Qian, Fei Zhao, Lei Shen, Gregory D Scholes, Mitchell A Winnik
Index: Langmuir 25(19) , 11732-40, (2009)
Full Text: HTML
Abstract
We report a method for preparing highly photoluminescent, water-soluble CdSe/CdS/ZnS core/shell/shell and CdSe/ZnS core/shell quantum dots (QDs) colloidally stabilized by double hydrophilic copolymers. The polymers, either a diblock copolymer poly(ethylene glycol-b-2-N,N-dimethylaminoethyl methacrylate) (PEG-b-PDMA) or a statistical copolymer poly(oligoethylene glycol methacrylate-co-2-N,N-dimethylaminoethyl methacrylate) (POEG-co-PDMA), were able to replace the hexadecylamine (HDA) or trioctylphosphine oxide (TOPO) ligands on the surface of the as-synthesized QDs and impart water-solubility and colloidal stability to the QD nanocrystals. In water, the [CdSe/ZnS]/POEG-co-PDMA colloids were present in the form of aggregates with a mean apparent hydrodynamic radius Rh of 54 nm and a narrow size distribution. Although the photoluminescence (PL) quantum yield (QY) of the polymer-treated QDs decreased upon transfer from an organic medium to water, much of this loss in brightness could be restored by the addition to the solution of an excess of a water-soluble primary amine such as 3-amino-propanol (APP). This chemical-activation strategy of adding primary amines as PL activators to polymer-stabilized QDs did not lead to a spectral shift of either the absorption or emission of the QDs in water.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
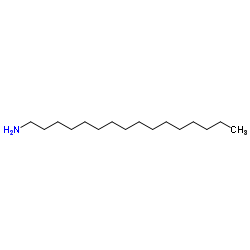 |
Hexadecylamine
CAS:143-27-1 |
C16H35N |
|
A carbon nanotube/poly [Ni-(Protoporphyrin IX)] composite fo...
2015-08-01 [Bioelectrochemistry 104 , 51-7, (2015)] |
|
Simple and sensitive progesterone detection in human serum u...
2015-08-15 [Anal. Biochem. 483 , 54-61, (2015)] |
|
Ketogenic response to cotreatment with bezafibrate and mediu...
2015-10-01 [Nutrition 31 , 1255-9, (2015)] |
|
Near-Room Temperature Synthesis of Core/Shell-Structured Qua...
2015-09-01 [J. Nanosci. Nanotechnol. 15 , 7146-52, (2015)] |
|
New Derivatives of Natural Acyclic Guanidine Alkaloids with ...
2015-07-01 [Nat. Prod. Commun. 10 , 1171-3, (2015)] |