| Structure | Name/CAS No. | Articles |
|---|---|---|
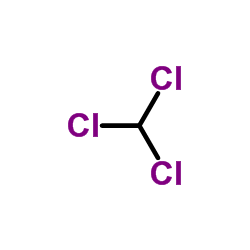 |
Chloroform
CAS:67-66-3 |
|
 |
1-Hexadecanol
CAS:36653-82-4 |
|
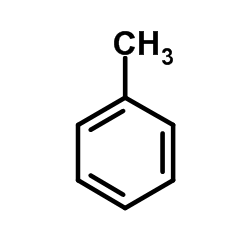 |
Toluene
CAS:108-88-3 |
|
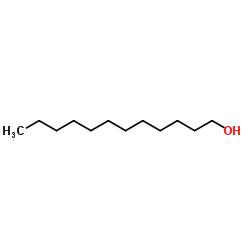 |
1-Dodecanol
CAS:112-53-8 |