| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Chloroform
CAS:67-66-3 |
|
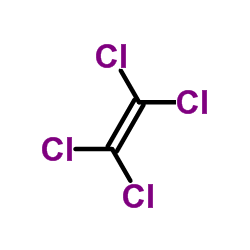 |
Tetrachloroethylene
CAS:127-18-4 |
|
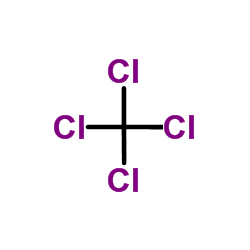 |
Carbon tetrachloride
CAS:56-23-5 |
|
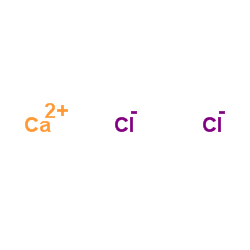 |
Calcium chloride
CAS:10043-52-4 |
|
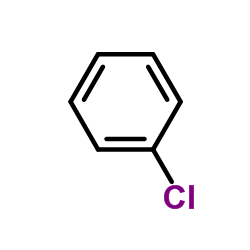 |
Chlorobenzene
CAS:108-90-7 |
|
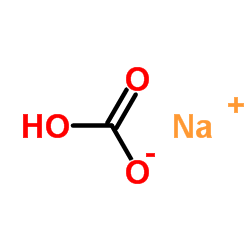 |
SodiuM bicarbonate
CAS:144-55-8 |
|
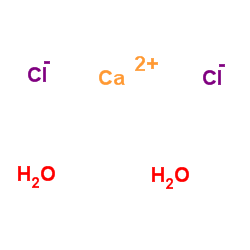 |
calcium chloride dihydrate
CAS:10035-04-8 |
|
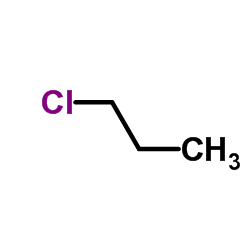 |
1-Chloropropane
CAS:540-54-5 |