| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Chloroform
CAS:67-66-3 |
|
 |
N-hexane
CAS:110-54-3 |
|
 |
n-Butyllithium
CAS:109-72-8 |
|
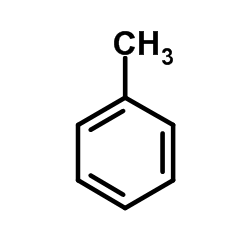 |
Toluene
CAS:108-88-3 |