|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~0% |
|
~81% |
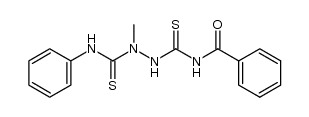
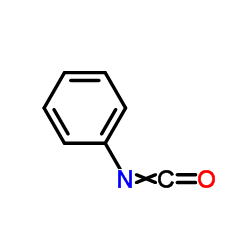


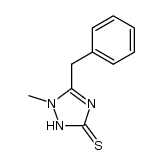
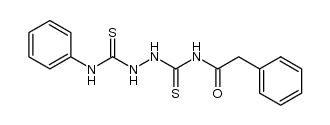
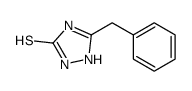


![N-[5-(methylthio)-1,3,4-thiadiazol-2-yl]benzamide Structure](https://image.chemsrc.com/caspic/189/5319-75-5.png)
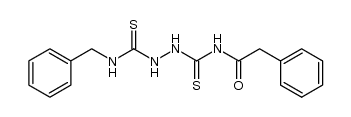
![N-[5-(benzylamino)-1,3,4-thiadiazol-2-yl]-2-phenylacetamide Structure](https://image.chemsrc.com/caspic/175/109853-22-7.png)