| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Chloroform
CAS:67-66-3 |
|
 |
L-γ-Glutamyl-S-nitroso-L-cysteinylglycine
CAS:57564-91-7 |
|
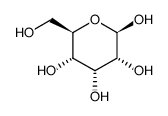 |
Beta-D-allose
CAS:7283-09-2 |