| Structure | Name/CAS No. | Articles |
|---|---|---|
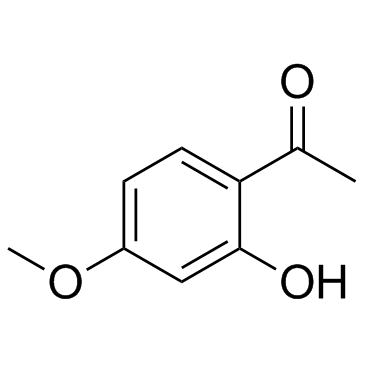 |
Paeonol
CAS:552-41-0 |
|
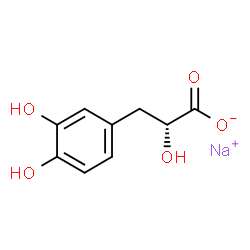 |
Danshensu Sodium Salt
CAS:81075-52-7 |