| Structure | Name/CAS No. | Articles |
|---|---|---|
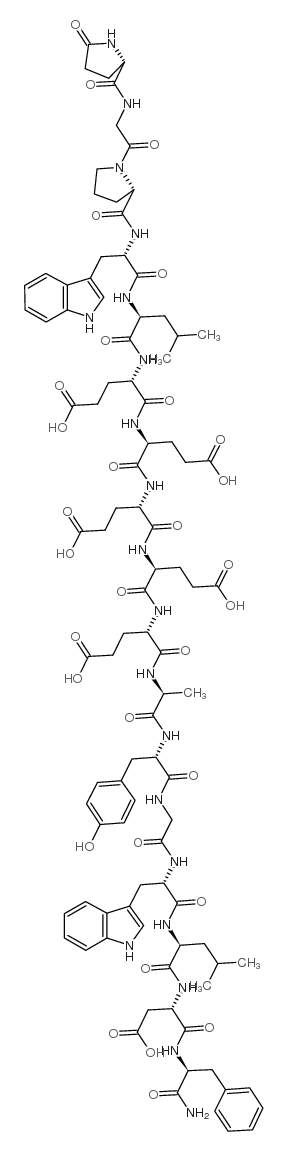 |
(Leu15)-Gastrin I (human)
CAS:39024-57-2 |
|
 |
4-(2-Isocyanoethyl)morpholine
CAS:78375-48-1 |