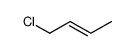
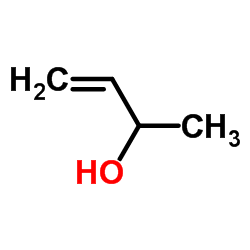
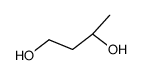
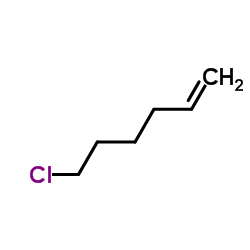
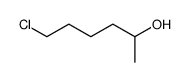
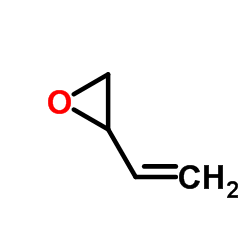
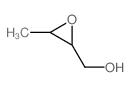
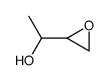
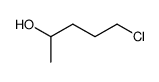
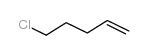
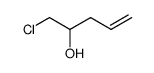
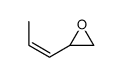
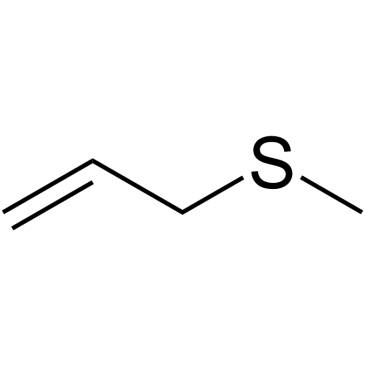
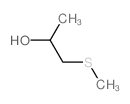
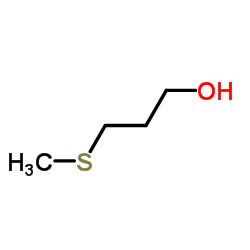
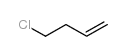
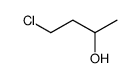
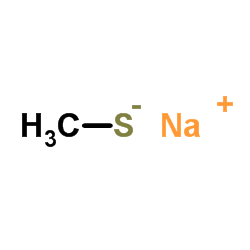|
~31%
Detail
|
|
~% |
|
~4% |
|
~81% |
|
~% |
|
~70% |
|
~% |
|
~81% |
|
~63% |