Robust calibration models to predict antipyrine content in various kinds of packaged hospital powder preparations by using near-infrared spectroscopy.
Makoto Otsuka, Mina Kamei
Index: Biomed Mater Eng 22(5) , 311-9, (2012)
Full Text: HTML
Abstract
The purpose of this study was to evaluate drug content of hospital powder preparations using non-destructive, non-contact, short-term measurements with near-infrared spectroscopy (NIR). Antipyrine (0-50%) was mixed with additive powder, and packed with semi-transparent (SP) or transparent (TP) paper to obtain single packaged samples (SPP and TPP). Double packaged samples were obtained by packing TPP with TP. NIR spectra of the packaged samples were recorded using a NIR spectrometer with a fiber-optic probe. The best calibration model was determined to minimize the standard error of cross-validation (SEP) by the leave-out-one method in principal component regression (PCR). The calibration models for SPP and TPP were calculated to be the minimum SEP based on one- and four-PC models by the PCR method, respectively. Plots of predicted and actual drug contents for SPP and TPP showed a straight line with a slope of 0.946 and 0.948, and γ of 0.965 and 0.980, respectively. On the other hand, the calibration model for the single and double TPP was obtained based on a three-PC model, and the plot of predicted and actual drug content showed a straight line with a slope of 0.942 and γ of 0.977.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
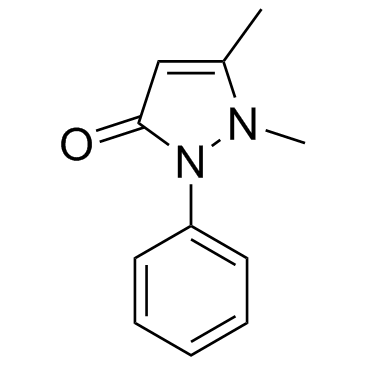 |
Antipyrine
CAS:60-80-0 |
C11H12N2O |
|
Calculating virtual log P in the alkane/water system (log P(...
2005-05-05 [J. Med. Chem. 48 , 3269-79, (2005)] |
|
The free radical scavenger, edaravone, ameliorates delayed n...
2013-01-01 [Undersea Hyperb. Med. 40(3) , 223-9, (2013)] |
|
Protective effect of edaravone against Alzheimer's disease-r...
2012-12-07 [Neurosci. Lett. 531(2) , 160-5, (2012)] |
|
Edaravone alleviates hypoxia-acidosis/reoxygenation-induced ...
2013-05-24 [Neurosci. Lett. 543 , 72-7, (2013)] |
|
Differential induction of cytochrome P450 isoforms and perox...
2013-07-04 [Toxicol. Lett. 220(2) , 135-42, (2013)] |