| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodiumborohydride
CAS:16940-66-2 |
|
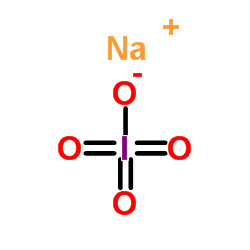 |
Sodium periodate
CAS:7790-28-5 |
|
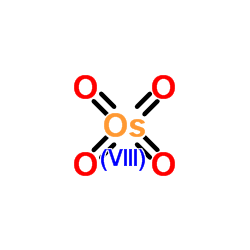 |
Osmium tetroxide
CAS:20816-12-0 |
|
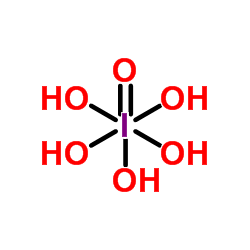 |
Pentahydroxy-λ5-iodane oxide
CAS:10450-60-9 |