| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
sodiumborohydride
CAS:16940-66-2 |
|
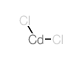 |
Cadmium chloride
CAS:10108-64-2 |
|
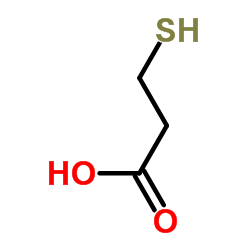 |
3-Mercaptopropionic acid
CAS:107-96-0 |