Study of interactions between food phenolics and aromatic flavors using one- and two-dimensional (1)H NMR spectroscopy.
D M Jung, J S de Ropp, S E Ebeler
Index: J. Agric. Food Chem. 48(2) , 407-12, (2000)
Full Text: HTML
Abstract
Changes in flavor release and aroma characteristics in a medium including food phenolics may be attributed to an intermolecular interaction between flavor compounds and phenolics. To investigate the interaction, one- and two-dimensional NMR studies have been carried out on the binding of two phenolics, gallic acid and naringin, with three aroma compounds, 2-methylpyrazine, vanillin, and ethyl benzoate. Evaluation of thermodynamic parameters and intermolecular nuclear Overhauser effects reveals that gallic acid can interact more strongly with aromatic flavors than naringin. The supramolecular complexation is also dependent on the structural nature of the flavors, with 2-methylpyrazine and vanillin interacting more strongly than ethyl benzoate. The interaction is principally pi-pi stacking between the galloyl ring and the aromatic ring of the aroma compounds, but secondary hydrogen-bonding effects help to stabilize the complex and enhance the specificity.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
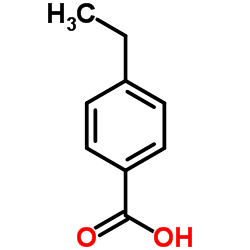 |
4-Ethylbenzoic acid
CAS:619-64-7 |
C9H10O2 |
|
Large-area graphene films by simple solution casting of edge...
2011-06-28 [ACS Nano 5(6) , 4974-80, (2011)] |
|
catena-Poly[[aqua-(4-ethyl-benzoic acid-κO)lanthanum(III)]-t...
2010-01-01 [Acta Crystallogr. Sect. E Struct. Rep. Online 66(Pt 2) , m183-4, (2010)] |
|
Anaerobic activation of p-cymene in denitrifying betaproteob...
2014-12-01 [Appl. Environ. Microbiol. 80(24) , 7592-603, (2014)] |
|
Redesigning metabolic routes: manipulation of TOL plasmid pa...
1987-01-30 [Science 235(4788) , 593-6, (1987)] |
|
Effect of tobacco smoke compounds on the plasma membrane of ...
1980-01-01 [Toxicology 15(3) , 203-17, (1980)] |