| Structure | Name/CAS No. | Articles |
|---|---|---|
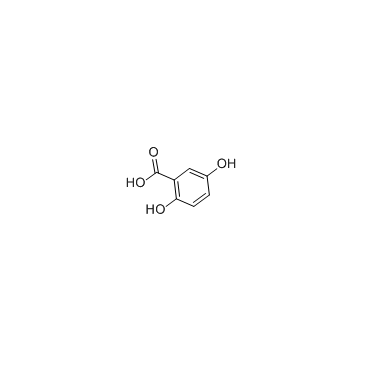 |
Gentisic acid
CAS:490-79-9 |
|
 |
Potassium bromide
CAS:7758-02-3 |
|
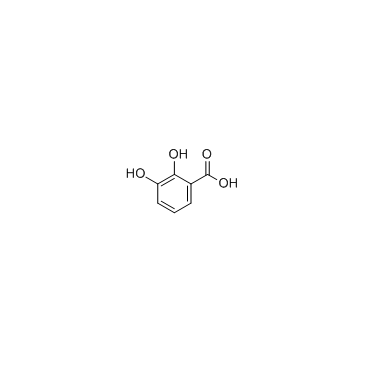 |
2,3-Dihydroxybenzoic acid
CAS:303-38-8 |
|
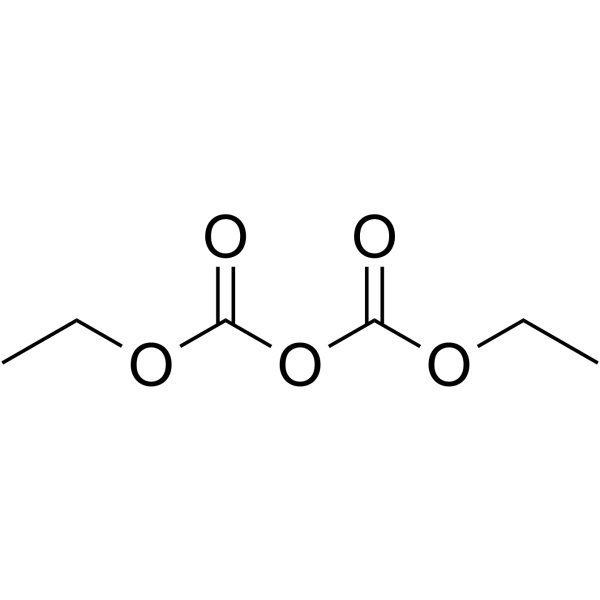 |
Diethyl pyrocarbonate
CAS:1609-47-8 |
|
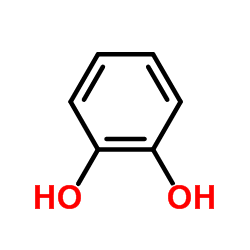 |
1,2-Benzenediol
CAS:120-80-9 |
|
 |
Deferoxamine (mesylate)
CAS:138-14-7 |
|
 |
DMPO
CAS:3317-61-1 |