Protein disulfide isomerase mediates integrin-dependent adhesion.
J Lahav, N Gofer-Dadosh, J Luboshitz, O Hess, M Shaklai
Index: FEBS Lett. 475(2) , 89-92, (2000)
Full Text: HTML
Abstract
Cell adhesion is mediated by the integrin adhesion receptors. Receptor-ligand interaction involves conformational changes in the receptor, but the underlying mechanism remains unclear. Our earlier work implied a role for sulfhydryls in integrin response to ligand binding in the intact blood platelet. We now show that non-penetrating blockers of free sulfhydryls inhibit beta(1) and beta(3) integrin-mediated platelet adhesion regardless of the affinity state of the integrin. Removal of the inhibitors prior to adhesion fully restores adhesion despite the irreversible nature of inhibitor-thiol interaction, indicating sulfhydryl exposure in response to adhesion. We further show that blocking protein disulfide isomerase (PDI) inhibits adhesion. These data indicate that: (a) ecto-sulfhydryls are necessary for integrin-mediated platelet adhesion; (b) disulfide exchange takes place during this process; (c) surface PDI is involved in integrin-mediated adhesion.
Related Compounds
| Structure | Name/CAS No. | Molecular Formula | Articles |
|---|---|---|---|
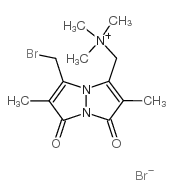 |
Monobromo(trimethylammonio)bimane bromide
CAS:71418-45-6 |
C13H19Br2N3O2 |
|
Cell surface thiols, but not intracellular glutathione, are ...
1985-10-01 [Immunol. Invest. 14(5) , 401-14, (1985)] |
|
The open/closed conformational equilibrium of aspartate amin...
1991-03-14 [Eur. J. Biochem. 196 , 329, (1991)] |
|
Thiol labeling with bromobimanes.
1987-01-01 [Meth. Enzymol. 143 , 76, (1987)] |
|
Localization of thiol and disulfide groups in guinea pig spe...
1984-11-01 [Biol. Reprod. 31(4) , 797-809, (1984)] |
|
Conformational changes in mitochondrial aspartate aminotrans...
1984-01-01 [Prog. Clin. Biol. Res. 144B , 117-24, (1984)] |