| Structure | Name/CAS No. | Articles |
|---|---|---|
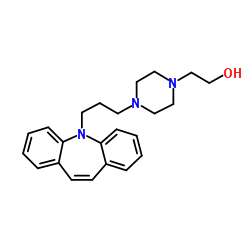 |
Gamma glutamyltransferase
CAS:9046-27-9 |
|
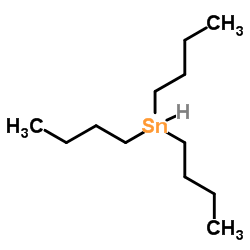 |
Tributyltin hydride
CAS:688-73-3 |
|
 |
GHRP-6 Acetate
CAS:87616-84-0 |