| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethidium bromide
CAS:1239-45-8 |
|
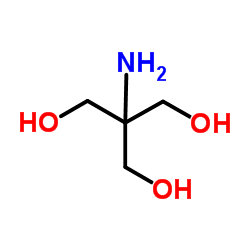 |
Trometamol
CAS:77-86-1 |
|
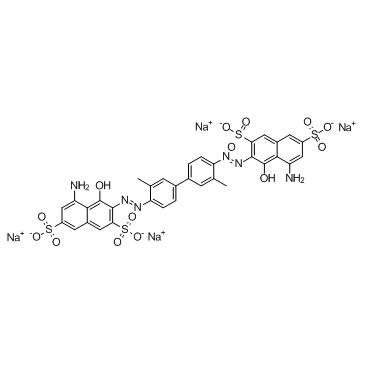 |
Direct Blue 14
CAS:72-57-1 |
|
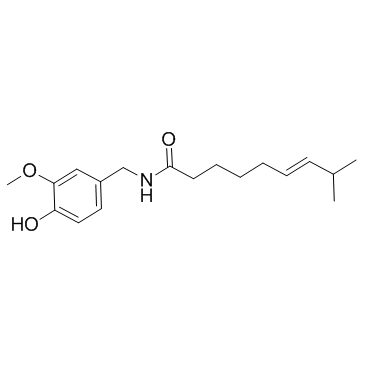 |
capsaicin
CAS:404-86-4 |
|
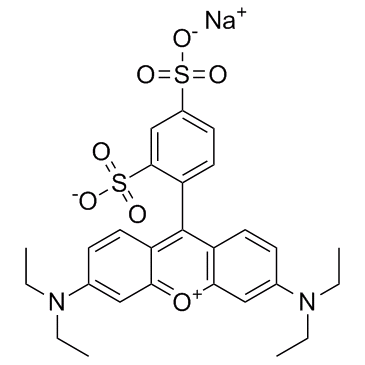 |
Acid Red 52
CAS:3520-42-1 |