| Structure | Name/CAS No. | Articles |
|---|---|---|
 |
Ethidium bromide
CAS:1239-45-8 |
|
 |
Chlorambucil
CAS:305-03-3 |
|
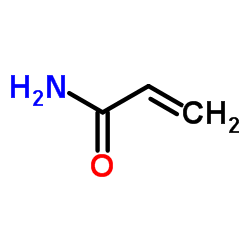 |
Acrylamide Crystals
CAS:79-06-1 |