| Structure | Name/CAS No. | Articles |
|---|---|---|
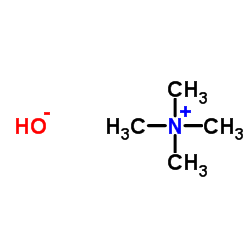 |
Tetramethylammonium hydroxide
CAS:75-59-2 |
|
 |
Sodium hydroxide
CAS:1310-73-2 |
|
 |
Methanol
CAS:67-56-1 |
|
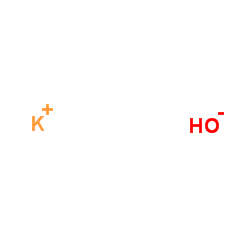 |
Potassium hydroxide
CAS:1310-58-3 |
|
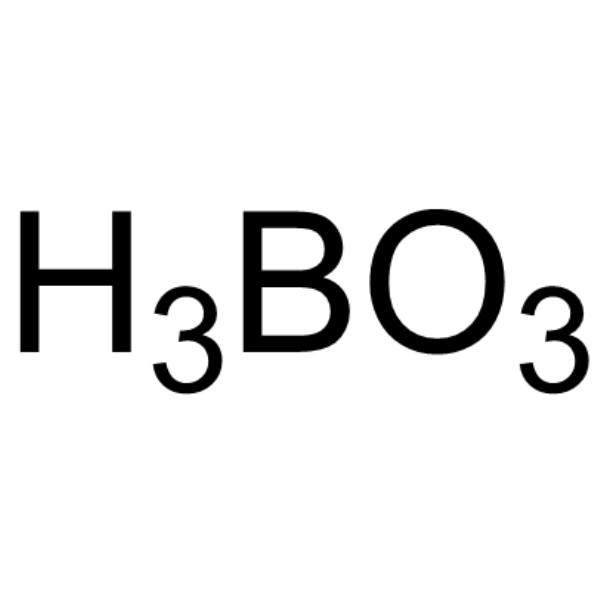 |
Orthoboric acid
CAS:10043-35-3 |
|
 |
Tetrabutylammonium hydroxide
CAS:2052-49-5 |
|
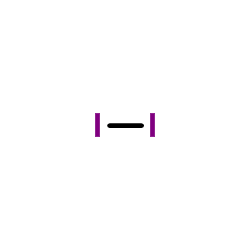 |
molecular iodine
CAS:7553-56-2 |
|
 |
Potassium iodide
CAS:7681-11-0 |
|
 |
3-Ethyl-2,4-pentanedione
CAS:1540-34-7 |
|
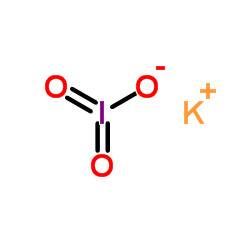 |
Potassium iodate
CAS:7758-05-6 |