| Structure | Name/CAS No. | Articles |
|---|---|---|
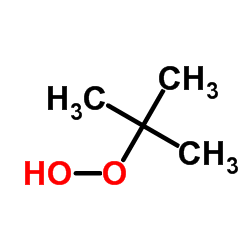 |
tert-Butyl Hydroperoxide
CAS:75-91-2 |
|
 |
Sulfuric acid
CAS:7664-93-9 |
|
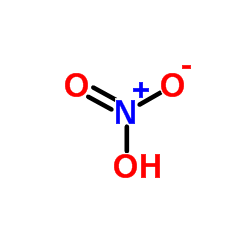 |
nitric acid
CAS:7697-37-2 |