|
~% |
|
~99% |
|
~% |
|
~% |
|
~97% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~72% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~93% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
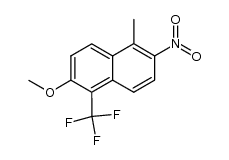

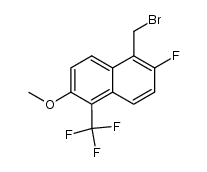
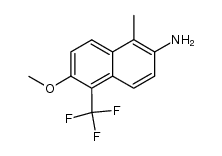

![N-[[2-fluoro-6-methoxy-5-(trifluoromethyl)-1-naphthalenyl]carbonyl]-N-(methoxycarbonyl)glycine tert-butyl ester Structure](https://image.chemsrc.com/caspic/437/122670-57-9.png)
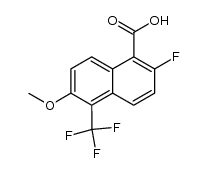
![N-[[2-fluoro-6-methoxy-5-(trifluoromethyl)-1-naphthalenyl]carbonyl]glycine tert-butyl ester Structure](https://image.chemsrc.com/caspic/268/122670-88-6.png)