|
~78% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
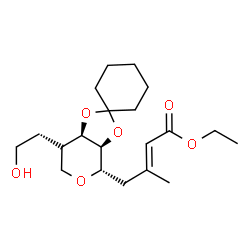



![(3a'S,7'R,7a'S)-7'-(2-((tert-butyldiphenylsilyl)oxy)ethyl)tetrahydro-4'H-spiro[cyclohexane-1,2'-[1,3]dioxolo[4,5-c]pyran]-4'-ol Structure](https://image.chemsrc.com/caspic/494/75452-41-4.png)
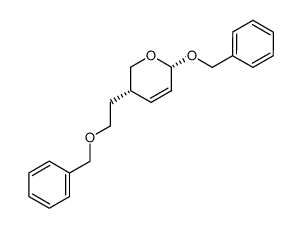
![ethyl (E)-4-((3a'S,4'S,7'S,7a'R)-7'-(2-((tert-butyldiphenylsilyl)oxy)ethyl)tetrahydro-4'H-spiro[cyclohexane-1,2'-[1,3]dioxolo[4,5-c]pyran]-4'-yl)-3-methylbut-2-enoate Structure](https://image.chemsrc.com/caspic/461/87641-25-6.png)