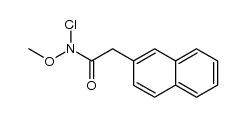
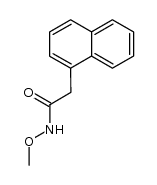
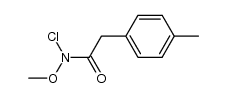
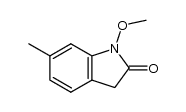
|
~98% |
|
~% |
|
~% |
|
~63% |
|
~% |
|
~61% |
|
~% |
|
~% |
|
~69% |
|
~71% |
|
~94% |
|
~97% |
|
~96% |
|
~84% |
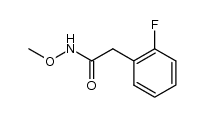
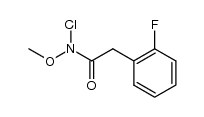
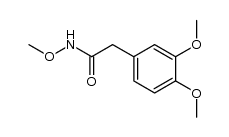
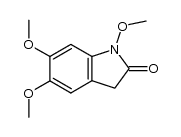
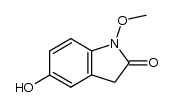
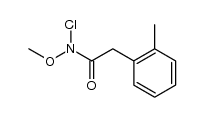
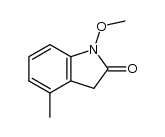


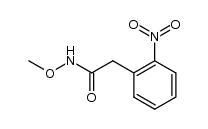

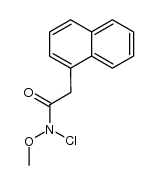
![1,3-dihydro-1-methoxy-2H-benz[c]indol-2-one Structure](https://image.chemsrc.com/caspic/051/113519-29-2.png)