|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~95% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
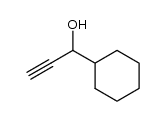
![4-[(1R,1α,6α,7Z)-2α-[(S)-3-Cyclohexyl-3-hydroxy-1-propynyl]-3β-hydroxybicyclo[4.2.0]octan-7-ylidene]butanoic acid Structure](https://image.chemsrc.com/caspic/238/105880-66-8.png)
![(S)-3-[(tert-Butyldimethylsilyl)oxy]-3-cyclohexyl-1-propyne Structure](https://image.chemsrc.com/caspic/339/105803-39-2.png)
![(1SR,2SR,4RS,7SR)-spiro(3-oxatricyclo[5.2.0.0]nonane-8,2'-[1,3]dioxolane) Structure](https://image.chemsrc.com/caspic/153/108147-26-8.png)
![(2R,3S)-2-((R)-3-((tert-butyldimethylsilyl)oxy)-3-cyclohexylprop-1-yn-1-yl)spiro[bicyclo[4.2.0]octane-7,2'-[1,3]dioxolan]-3-ol Structure](https://image.chemsrc.com/caspic/228/105880-44-2.png)
![(3'S,1S,2S,3R,6S)-2-(3'-cyclohexyl-3'-hydroxyprop-1'-ynyl)-3-hydroxybicyclo[4.2.0]octan-7-one Structure](https://image.chemsrc.com/caspic/408/105803-42-7.png)
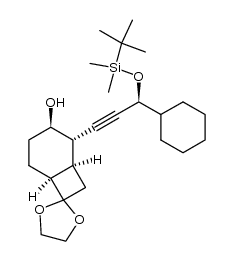

![(1R,2S,3R,6S)-2-((S)-3-((tert-butyldimethylsilyl)oxy)-3-cyclohexylprop-1-yn-1-yl)spiro[bicyclo[4.2.0]octane-7,2'-[1,3]dioxolan]-3-yl ((R)-1-(naphthalen-1-yl)ethyl)carbamate Structure](https://image.chemsrc.com/caspic/127/105881-33-2.png)
![(E)-4-((1S,2S,3R,6S)-2-((S)-3-cyclohexyl-3-hydroxyprop-1-yn-1-yl)-3-hydroxybicyclo[4.2.0]octan-7-ylidene)butanoic acid Structure](https://image.chemsrc.com/caspic/173/105880-50-0.png)