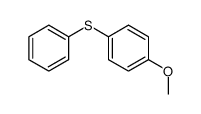
|
~28% |
|
~41% |
|
~48% |
|
~56% |
|
~5% |
|
~88% |
|
~86% |
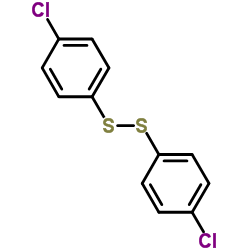
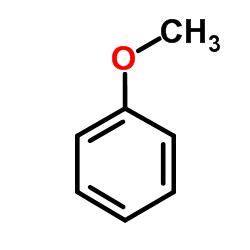



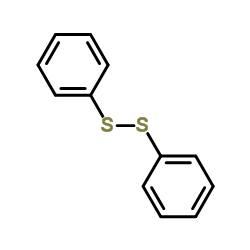
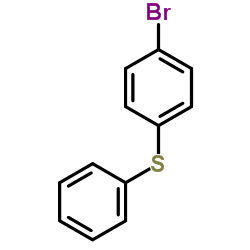

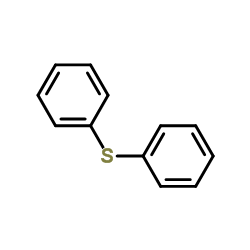
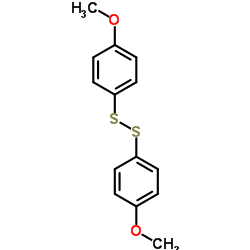
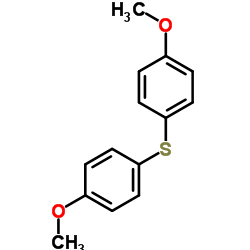
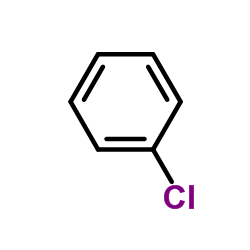
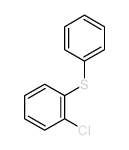
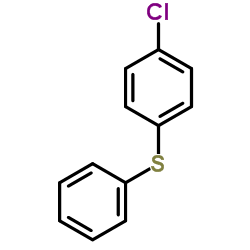
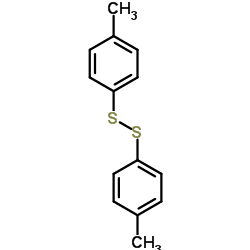
![2-[N-(benzenesulfonyl)-2-ethoxyanilino]-N-cyclopentylacetamide Structure](https://image.chemsrc.com/caspic/455/6013-47-4.png)
![1-methoxy-2-[(4-methylphenyl)thio]-benzene Structure](https://image.chemsrc.com/caspic/108/59345-33-4.png)