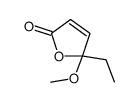
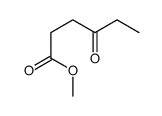
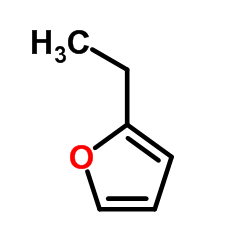
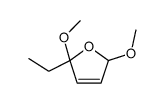
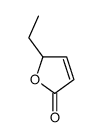
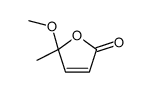
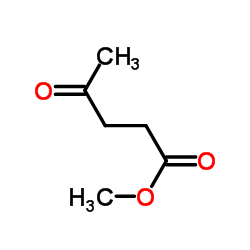
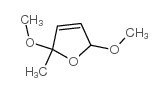
|
~% |
|
~% |
|
~% |
|
~87% |
|
~% |
|
~% |