|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
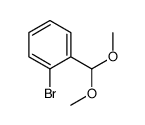
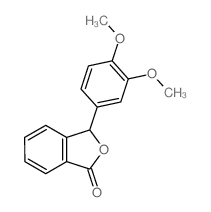
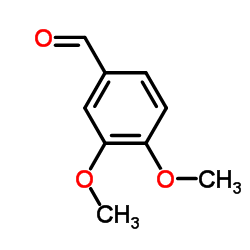
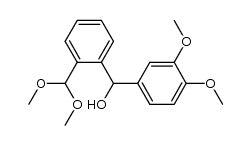
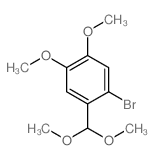

![benzo[d][1,3]dioxol-5-yl(2-(dimethoxymethyl)-4,5-dimethoxyphenyl)methanol Structure](https://image.chemsrc.com/caspic/444/74879-19-9.png)
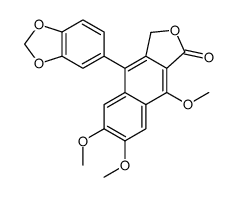
![4-benzo[1,3]dioxol-5-yl-9-hydroxy-6,7-dimethoxy-3H-naphtho[2,3-c]furan-1-one Structure](https://image.chemsrc.com/caspic/308/17803-11-1.png)
