|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
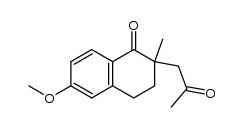
![7-methoxy-3a-methyl-4,5-dihydro-3H-cyclopenta[a]naphthalen-2-one Structure](https://image.chemsrc.com/caspic/248/27752-28-9.png)
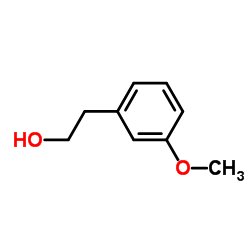

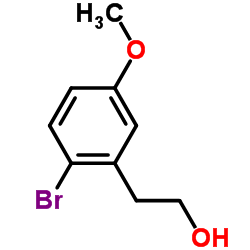
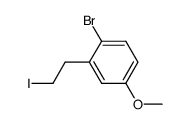
![(3S,6R,7aR)-6-(2-bromo-5-methoxyphenethyl)-3-isopropyl-6,7a-dimethyltetrahydropyrrolo[2,1-b]oxazol-5(6H)-one Structure](https://image.chemsrc.com/caspic/348/153683-09-1.png)