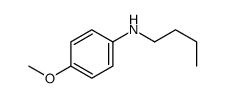
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~86% |
|
~% |
|
~% |
|
~90% |
|
~% |
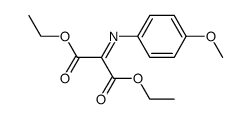

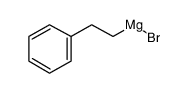


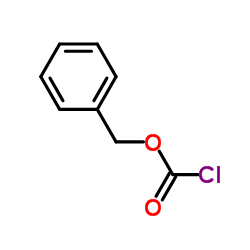
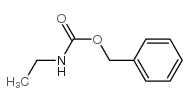
![diethyl 2-[dodecyl(p-methoxyphenyl)amino]malonate Structure](https://image.chemsrc.com/caspic/166/369363-26-8.png)
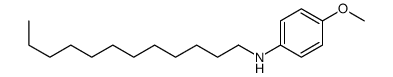
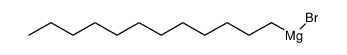
![diethyl 2-[butyl(p-methoxyphenyl)amino]malonate Structure](https://image.chemsrc.com/caspic/242/369363-24-6.png)