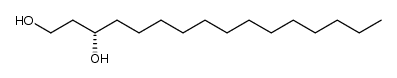
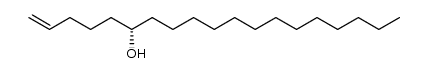
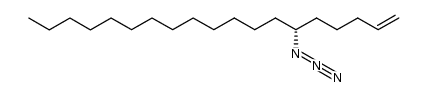
|
~% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~86% |
|
~% |
|
~% |
|
~% |
|
~% |
|
~91% |
|
~% |
|
~78% |
![4,7,7-trimethyl-3-exo-(1-naphthyl)bicyclo[2.2.1]hept-2-exo-yl 3-oxohexadecanoate Structure](https://image.chemsrc.com/caspic/148/114634-26-3.png)
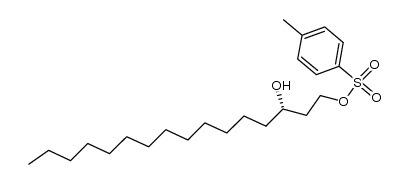
![(S)-(1S,2R,3R,4S)-4,7,7-trimethyl-3-(naphthalen-1-yl)bicyclo[2.2.1]heptan-2-yl 3-hydroxyhexadecanoate Structure](https://image.chemsrc.com/caspic/110/114634-27-4.png)