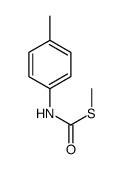
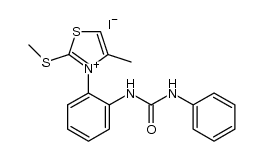
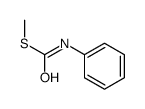
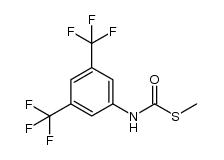
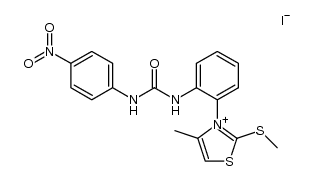
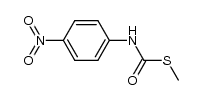
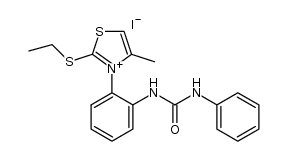
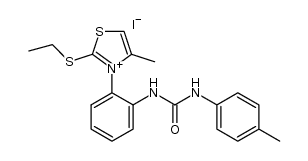
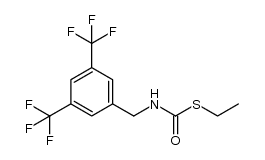
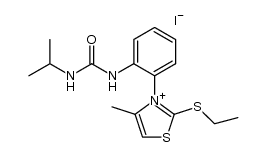
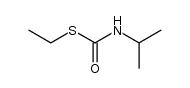
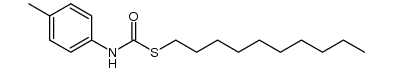
|
~91% |
|
~92% |
|
~96% |
|
~95% |
|
~95% |
|
~97% |
|
~92% |
|
~95% |
|
~97% |
|
~96% |
|
~97% |

![Thiazolo[3,2-a]benzimidazole, 3-methyl- (7CI,8CI,9CI) Structure](https://image.chemsrc.com/caspic/390/5268-73-5.png)