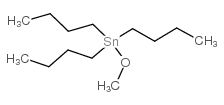
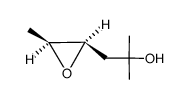
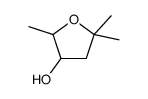
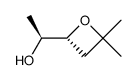
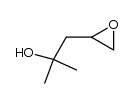
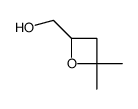
|
~% |
|
~% |
|
~% |
|
~% |
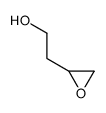

![tributyl-[2-(oxiran-2-yl)ethoxy]stannane Structure](https://image.chemsrc.com/caspic/458/61266-51-1.png)