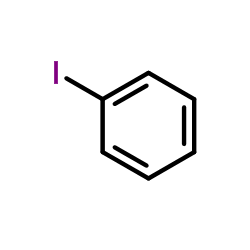
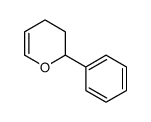
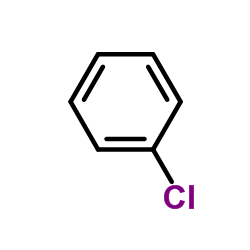
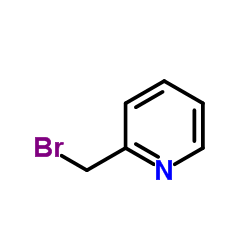
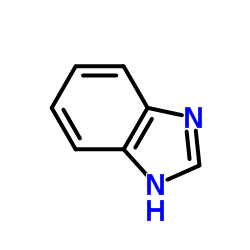
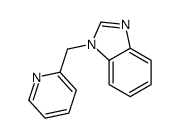
|
~51% |
|
~34% |
|
~5% |
|
~81% |